During aging, our tissues become crosslinked.
Crosslinks are connections (“links”) formed between the building blocks that make up our tissues.
For example, sugar molecules are able to form crosslinks between proteins like collagen, which is an important component of our skin or blood vessels.
These sugar crosslinks are called AGEs, which stands for Advanced Glycation End products.
When lots of our collagen is crosslinked or “glued together” by sugar crosslinks (AGEs), tissues become more stiff.
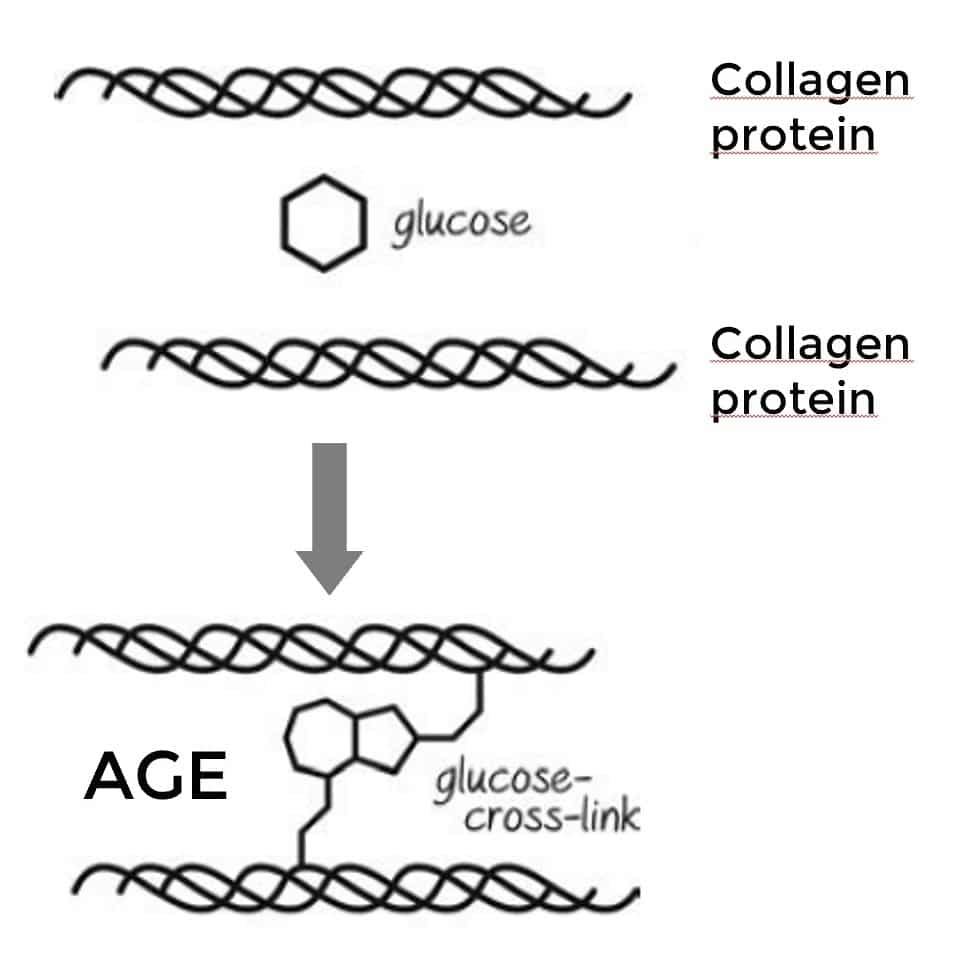
Glucose forms a crosslink between two collagen proteins, creating an advanced glycation end product (AGE).
Crosslinking of collagen and elastin proteins in the skin is one of the reasons why the skin becomes less flexible and more stiff and wrinkly.
Crosslinking of the collagen and elastin in our blood vessels makes them more stiff, which leads to “hardened” blood vessels, increased blood pressure (hypertension), and makes blood vessels more prone to break, which can cause a bleeding in the brain (a stroke) or other organs like the gut.
Crosslinking of proteins in the lungs makes the lungs more stiff, resulting in a decline in lung function. Crosslinking also makes the lungs more prone to infections, which explains why elderly persons have a much higher risk of dying of pneumonia and other lung infections.
Crosslinks occur everywhere in nature. One reason why tree bark is so hard is because of crosslinking. Also, when you toast bread in the kitchen, it becomes “hard” and brownish due to sugar-crosslinks forming between the proteins that make up the bread. The same process of crosslinking gives your chicken a nice hard brown crust when cooking it.
Interestingly, consuming crosslinks (like via toasted bread or via heated meats) can also damage the body. There are receptors on our cells that are activated by crosslinks (these receptors are called RAGEs – Receptors for Advanced Glycation End products). When these are activated by crosslinks, all rage breaks lose indeed, given they induce inflammation in cells for example.
Different types of crosslinks
There exist different kinds of crosslinks in the body, such as:
- Glucosepane: this is the most common crosslink in our tissues
- Pentosidine
- Carboxymethyl-lysine (CML)
- Carboxyethyl-lysine (CEL)
- Pyrraline
- Many other types of crosslinks
Lots of crosslinks are based on sugars, hence the name Advanced Glycation End products (AGEs). However, there exist also crosslinks or unwanted modifications of proteins based on fats instead of sugars, which are called “Advanced Lipoxidation End products” or ALEs.
Other modifications of proteins: glycation, carbamylation and citrullination
Besides crosslinks, other detrimental changes are made to proteins, which involve chemical reactions that put unwanted groups of molecules on proteins, damaging the proteins, or making proteins more susceptible to attacks by the immune system.
Depending on the type of molecule put on the proteins, scientists speak of glycation, carbamylation, citrullination, or carbonylation.
Glycation means that a glucose molecule reacts with proteins, attaching itself to the protein. But it doesn’t connect with another protein, forming a crosslink (or an AGE). So glycation could be considered as the start of a crosslink, but does not always form a crosslink; it’s just a sugar attaching itself to the protein.
Lot’s of glycation means that many proteins have sugars attached to them. This hinders the proper functioning of the proteins, and also can lead to tissue stiffness for example.
Doctors measure glycation of the hemoglobin protein in red blood cells (called “HbA1c”) to measure how bad diabetes is in a person. When diabetes is not well controlled, lot’s of sugars circulate in the body, so there is a lot of glycation (and crosslinking) of proteins in the body, including of hemoglobin.
Small sugars like glucose, fructose and galactose are the main drivers of glycation. Fructose has ten times more glycation activity than glucose. This is interesting to know given that lots of unhealthy, sugary foods (like soda) contain “high fructose corn syrup”, which contains lots of fructose.
Glycation plays a role in diabetes, Alzheimer’s and heart disease (R,R).
Carbamylation involves a cyanate group (HNCO) reacting with a protein (specifically with the lysine amino acid in the protein), putting a homocitrulline group on the protein.
Carbamylation has been involved in inflammation, atherosclerosis and kidney diseases.
Citrullination involves a chemical modification in which a citrulline group is formed on the protein (specifically on the arginine amino acid in the protein; this chemical modification is done by an enzyme called “peptidylarginine deiminase” or PAD).
Citrullination has been associated with many diseases, like autoimmune diseases, cancer and neurodegenerative diseases.
How are these processes involved in autoimmune diseases? By putting these unwanted molecules on proteins, the structure of the proteins is somewhat changed. This causes the immune system to not recognize the proteins anymore, thinking they are “foreign” and attacking these altered proteins.
Citrullination and carbamylation play an important role in auto-immune diseases such as rheumatoid arthritis, in which the immune system attacks collagen proteins (R). Measuring the amount of citrullination in the body is in fact a medical test to help diagnose rheumatoid arthritis.
An unhealthy lifestyle, like eating sugary junk food or smoking, leads to more glycation, carbamylation, citrullination and other unhealthy modifications of our proteins, increasing the risk of auto-immune diseases, inflammation and accelerating aging (R,R).
Other changes to proteins
Besides crosslinking and chemical modifications like carbamylation, other processes also lead to hardening or stiffening of our tissues, such as accumulation of proteins (e.g. accumulation of proteins inside the artery walls or lungs makes them more stiff), or elastocalcinosis, which is the calcification of the elastic fibers in our tissues, especially in the blood vessels, making them more stiff.
Crosslinking in diabetes patients
As mentioned before, glycation and crosslinks are caused by sugar molecules sticking to proteins, and glycating or crosslinking them. People with type 2 diabetes often have high levels of glucose circulating in their body. This drastically increases the glycation and crosslinking in their blood vessels, kidney, nerves and other tissues, contributing to a dramatically increased risk of heart disease, kidney failure, nerve damage, neuropathy and eye problems (R).
Learn more about the hallmarks of aging:
Epigenetic alterations
Loss of proteostasis
Mitochondrial dysfunction
Genomic instability
Telomere attrition & shortening
Advanced Glycation End Products (AGEs) and protein modifications
Senescent cells
Stem cell exhaustion
Altered intercellular communication
Deregulated nutrient sensing
Other reasons why we age
Overview of the hallmarks of aging