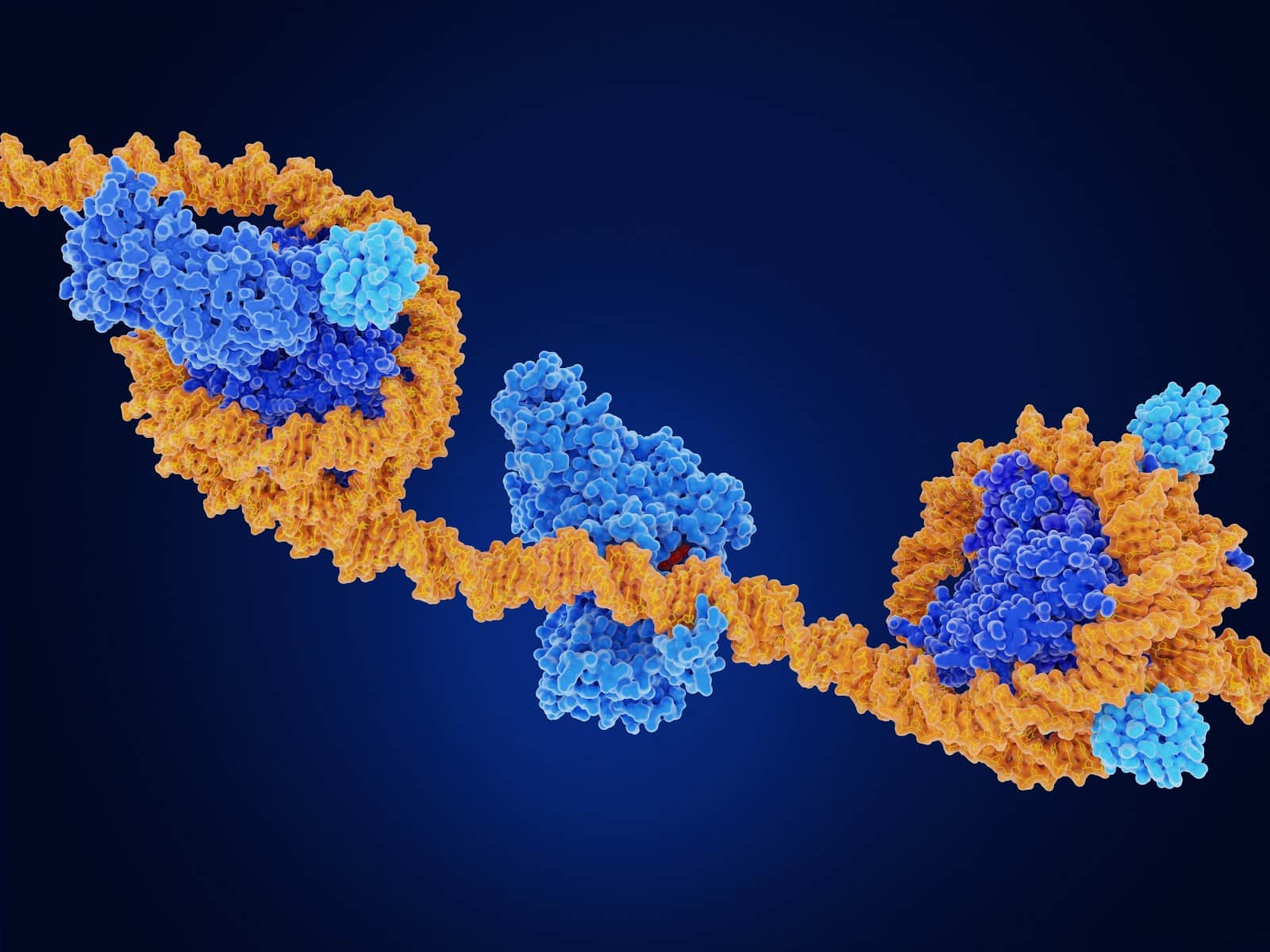
DNA strands (yellow) are wrapped around histone proteins (blue).
The epigenome plays an important role in aging. The epigenome determines which genes are switched on or off.
As we get older the epigenome becomes more dysregulated, leading to genes that are switched on that should be switched off, like pro-cancer or pro-inflammatory genes. And vice versa: aging leads to genes being switched off that should be switched on, like repair or housekeeping genes.
All this activation of the wrong genes, and silencing of good, healthy genes leads to our cells functioning less well. Given the importance of the epigenome in aging, let us go a bit deeper into the epigenome.
What is the epigenome?
The epigenome consists of 3 “layers” or parts:
DNA methylation
One way the epigenome can switch off genes is by methylating them. A gene is a part of our DNA. When methyl groups are placed on DNA, a gene is switched off. Methyl groups are small molecules (consisting of a carbon atom and three hydrogen atoms). When a gene is covered by lots of methyl groups, the cellular machinery (specific proteins) cannot reach this gene anymore to translate it into proteins (proteins are the building blocks and machinery of our cells). DNA methylation can also switch on genes by methylating a gene or region of the DNA that otherwise would switch off another gene.
Histonlyation
Histones are little balls of proteins around which our DNA strands are winded. Just like yearn is rolled up around a spool or bobbin, histones are the proteins around which DNA strands are winded. If DNA strands are tightly rolled up around histones, then the genes that are part of this DNA cannot be accessed by the gene-reading machinery, effectively silencing or inactivating those genes.
Chromatin
Chromatin is all the DNA in our cell. It’s how the DNA appears when looking at the cell nucleus – the nucleus contains the DNA. The DNA on a chromatin level is condensed (wrapped up) in specific ways. For example, it can form chromosomes, which are large structures of condensed DNA.
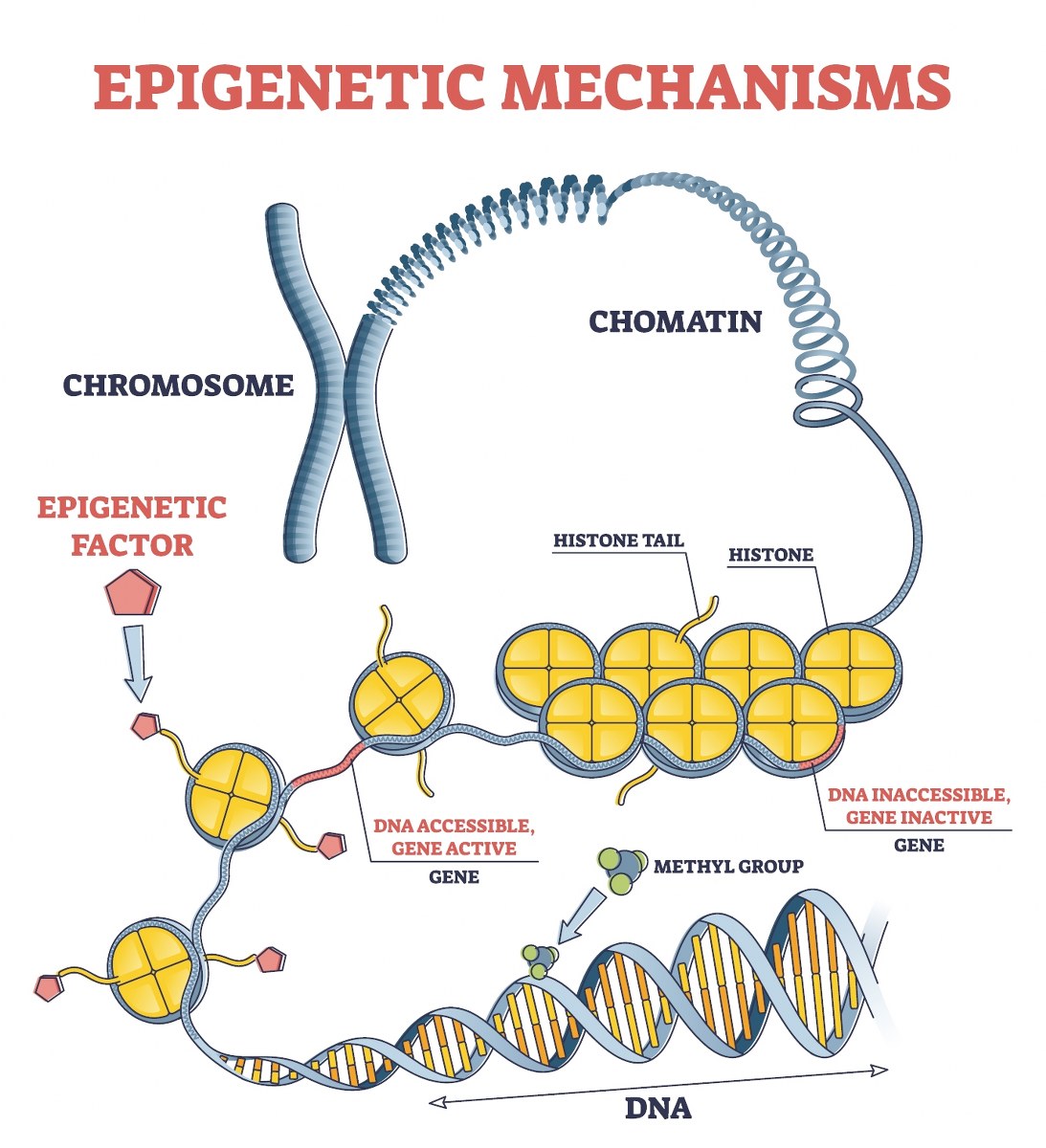
A DNA strand (starting at the bottom) is methylated and then wrapped around histone proteins (yellow structures), which further condense into chromatin, forming chromosomes.
The organization of the epigenome is a very impressive feat. The DNA strand in our cells is around 2 meters (or yards) long! So a 2-meter-long strand of DNA needs to be folded in such a way it can fit in a tiny nucleus with a diameter of only 10 micron (a micron is a thousandth of a millimeter).
However, by rolling up the DNA around millions of histones and then organizing these histones and strands our cells manage to fit 2 meters of DNA into the minuscule cell nucleus. Even more, our cells can duplicate all this DNA with relatively little errors during each cell division, creating two new cells with each 2 meters of DNA.
If we would unravel the DNA from all our cells in our body (about 40 billion cells) and string this DNA together into one big strand, it would stretch for about 67 billion miles or 108 billion kilometers, or twice the diameter of our solar system. We contain a lot of DNA!
The epigenome is very important for the proper functioning of our cells.
After all, all our cells have the same DNA (except for red blood cells, they don’t have a cell nucleus containing DNA).
So despite our cells having the same DNA, there are around 200 different types of cells in our body, such as muscle cells, neurons, gut cells, liver cells, skin cells, and so on.
All these cells contain the same DNA, or the same set of instructions to build about 20,000 different proteins, which carry out most of the functions of the cell.
In other words, a liver cell is a liver cell and not a muscle cell or nervous cell, despite having the same DNA and same genes.
A liver cell is a liver cell because the epigenome switches off all genes that the liver cells do not need, like heart cell genes or stomach cell genes and thousands of other genes.
The same in other cells. The epigenome in skin cells makes sure the skin-cell genes are active, while the epigenome in brain cells turns on the brain-related genes (and switches off the skin, gut, or liver-related genes).
So the epigenome determines the identity of each cell, and by this also its fate.
After all, some cells live much longer than other cells. For example, brain cells can become as old as a human, like 80 years or older, whereas a skin cell only lives for about 4 weeks. This despite that brain cells and skin cells have the same DNA! It’s the epigenome that determines not only how cells look, but that also determines their lifespan and fate.
Aging and the epigenome
Given the enormous amount of (tight) organization of the epigenome, it’s not surprising that as the years of our lives pass this system becomes more and more dysregulated, contributing to aging.
This dysregulation of the epigenome leads to “bad” genes being turned on which normally should be turned off, like cancer-promoting genes (increasing the risk of cancer as we get older), pro-inflammatory genes and other genes that impede the proper functioning of the cells.
The opposite is also happening: during aging, “good”, beneficial genes are switched off, like repair genes, housekeeping genes, anti-inflammatory genes, and many other genes that keep our cells healthy and young.
DNA damage and epigenetic dysregulation
It’s possible that DNA damage (another cause of aging) can also cause epigenetic dysregulation.
When our DNA is damaged, the epigenome needs to unwind the DNA strand with the damage to allow proteins to access the DNA to repair it. Recall that most of the DNA is wound up, so it needs to be unwinded or loosed up in order for various DNA repair proteins to reach the damaged spot and repair the damage.
This causes the epigenome to become less maintained, because the proteins involved in DNA repair also maintain the epigenome. But when these proteins are busy unwinding and repairing the DNA damage, they cannot preserve the epigenome.
Also, these proteins involved in DNA repair (and epigenetic maintenance) are not always neatly going back to their regular places to properly maintain the epigenome , which further could lead to deterioration of the epigenome (R,R).
In one study, mice were genetically modified to get lots of double strand breaks (a severe form of DNA damage)(R). However, all their double strand breaks were quickly repaired. However, they still aged a lot faster and looked much older than normal mice. Despite having an “intact”, undamaged genome. The researchers speculate that DNA damage does not cause aging directly (the DNA of the mice is intact), but that the epigenetic dysregulation that follows DNA damage does (R).
This could also explain why we all age quite “similarly”, despite that everyone can have mutations in different parts of their DNA which could lead to quite different ways of aging. It’s not that some persons can live 200 years, or age very differently (e.g. looking extremely young or old for their age). Perhaps it’s not so much the DNA damage that is important in aging, but the epigenetic consequences this damage has, disrupting large parts of our genome given the DNA is not properly folded and epigenetically maintained anymore.
Conclusion
Whatever the causes may be, malfunction of the epigenome is an important contributor to aging.
Luckily, various substances exist that can help to better maintain the epigenome, and scientists are exploring ways or reprogramming the epigenome back into a younger, more youthful state.
Learn more about the hallmarks of aging:
Epigenetic alterations
Loss of proteostasis
Mitochondrial dysfunction
Genomic instability
Telomere attrition & shortening
Advanced Glycation End Products (AGEs) and protein modifications
Senescent cells
Stem cell exhaustion
Altered intercellular communication
Deregulated nutrient sensing
Other reasons why we age
Overview of the hallmarks of aging