As we age, senescent cells arise everywhere in our body.
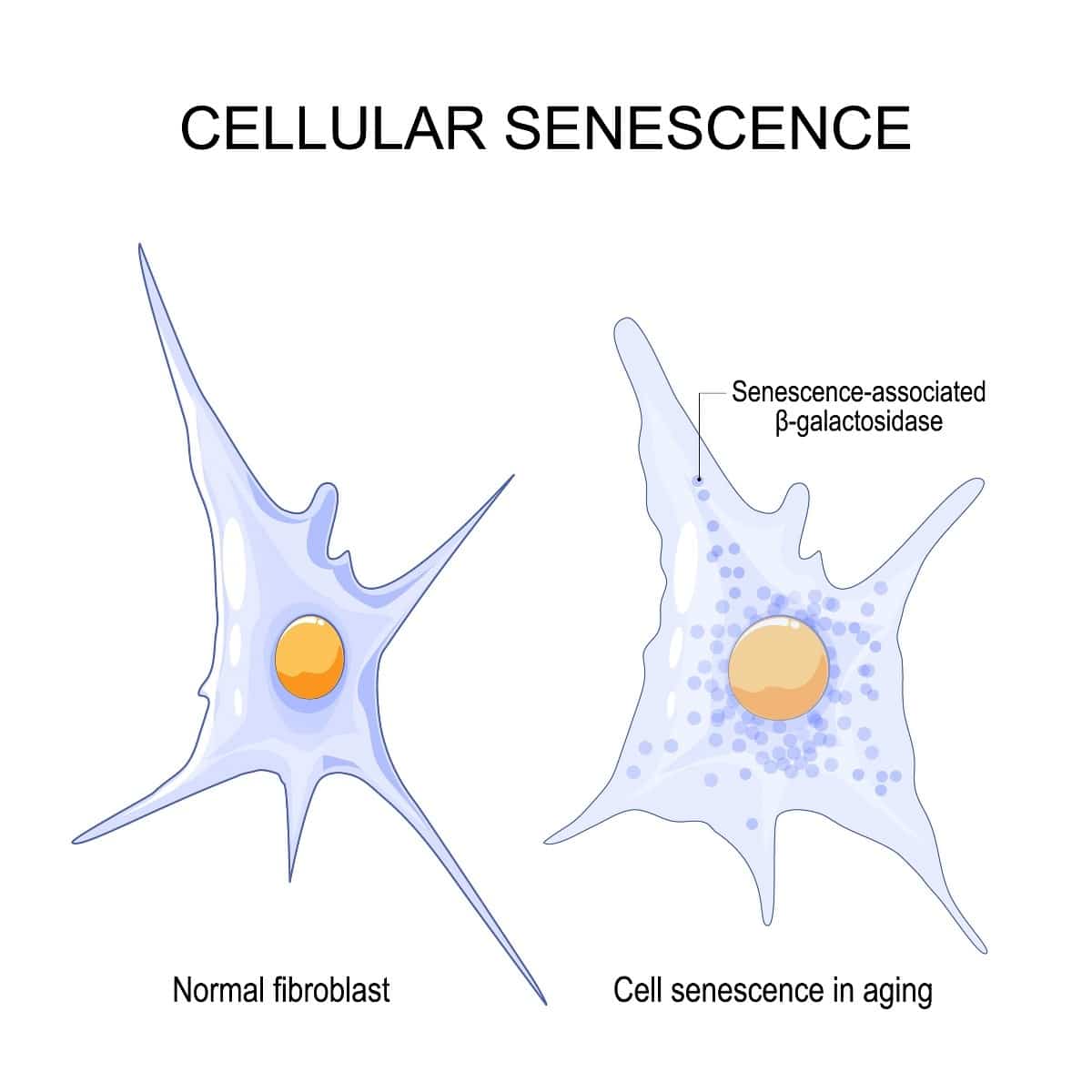
Senescent cells are cells that are damaged but refuse to die. Despite their damage, and their malfunctioning, they keep lingering on. Some scientists call them “zombie” cells, because they normally should be dead but they keep living on.
Senescent cells are very damaging to their environment. They secrete numerous substances that promote inflammation or malfunctioning in neighboring cells and tissues. The pro-inflammatory, damaging substances senescent cells secrete can even travel far and wide via the blood to other tissues, causing inflammation or damage everywhere in the body.
Examples of detrimental substances that senescent cells secrete are pro-inflammatory molecules like IL-6 (interleukin 6), IL-1, IL-8, TNF-alpha.
Senescent cells also secrete pro-coagulation substances that can make the blood coagulate faster.
They also secrete substances that break down the extracellular matrix, which is the gel-like glue that glues together all our cells. A more loose extracellular matrix makes other cells behave not as they should (like stem cells, which need a tightly regulated extracellular matrix to be embedded in), or makes it for cancer cells much easier to spread (metastasize). A damaged extracellular matrix also makes the tissues less elastic and plump, which plays a role in skin wrinkling, and weakened blood vessels.
Senescent cells originate everywhere in our body during aging. Senescent cells in the skin contribute to wrinkles. Senescent cells in the blood vessels contribute to atherosclerosis (the clogging up of our blood vessels). Senescent cells in the lungs make the lung tissue more stiff and prone to infections (such as pneumonia).
Also stem cells can become senescent. Senescent stem cells are less able to generate new cells to replenish our tissues, contributing to a decline of these tissues.
In other words: senescent cells are bad. They play a major role in aging.
Where do senescent cells come from?
How do senescent cells arise? They mainly arise due to damage they accrue.
Normally, if a cell becomes too damaged, it kills itself.
However, senescent cells evaded this, and they keep existing. Also, normally, the immune system clears away lots of senescent cells, but during aging the immune system becomes less able to clear senescent cells.
Senescent cells arise due to aging-related damage, caused by the aging-mechanisms we discussed before, such as epigenetic dysregulation, DNA damage, mitochondrial dysfunction, proteotoxic stress (due to protein accumulation) and so on. So fundamental aging processes contribute to the formation of senescent cells.
Senescent cells can also be caused by other kinds of damage, like exposure to UV light, radiation, toxic substances, including some medications, and so on.
How to clear senescent cells?
Scientists are trying to find ways to kill senescent cells.
One method is by creating a peptide that “frees” up an important protein called p53 (to be more precise, the peptide prevents p53 binding to FOXO4, another protein, which inhibits p53).
P53 is an “alarm” protein that kicks into action when a cell becomes too damaged. When p53 is activated by cell damage, three main outcomes can happen:
- The DNA damage is repaired (when there is not too much damage)
- The cell becomes senescent (when there is more damage), or
- The cell kills itself (when there is too much damage)
By liberating p53, senescent cells are killed. Destroying senescent cells this way resulted in some aspects of rejuvenation in mice (R): it restored fur density, fitness and renal function in both fast aging mice and in naturally aging mice.
Other ways to clear senescent cells is via small molecules (drugs) that target anti-apoptotic pathways (these pathways prevent senescent cells from killing themselves), or by attaching a senolytic drug to an molecule that attaches to receptors found on senescent cells), or by creating senolytic vaccines, which are substances that want to induce the immune system to specifically kill senescent cells (R).
An important aspect to clearing senescent cells, is making sure this intervention is sufficiently “specific”, meaning it kills only senescent cells, not healthy cells. Many small molecules that are called “senolytics” are not specific though, in the sense they can also damage healthy normal cells, including stem cells. So with senolytics, one has to be careful to not overdose or use substances that can damage healthy, non-senescent cells too.
An example of such dirty “senolytics” could be quercetin. This supplement has been shown to also kill stem cells. In fact, in some settings quercetin is actually used to kill remaining stem cells in batches of stem cells that got converted into normal, differentiated cells, to make sure no stem cells remain.
In other words, I would be cautious about taking high amounts of such “senolytics” to “clear” senescent cells, given they could also in such high doses damage normal healthy cells.
Learn more about the hallmarks of aging:
Epigenetic alterations
Loss of proteostasis
Mitochondrial dysfunction
Genomic instability
Telomere attrition & shortening
Advanced Glycation End Products (AGEs) and protein modifications
Senescent cells
Stem cell exhaustion
Altered intercellular communication
Deregulated nutrient sensing
Other reasons why we age
Overview of the hallmarks of aging