Slowing down aging is one thing, but reversing aging is something completely different.
“Reversing aging” means making old people young again. This would be far more interesting than just “slowing down aging”.
In the last few years, scientists discovered it’s possible to partially reverse the aging process. This means they could make old organisms younger again (learn more about the causes of aging here).
These studies show that aging is not a one-way street, but can be reversed. They show that aging is a plastic process, and that the “information to stay young” is still present in our body even when it’s old.
1. Reversing aging via epigenetic reprogramming
Currently, one of the most promising approaches to reverse aging is “epigenetic rejuvenation” or “epigenetic reprogramming”.
It’s based on a pioneering discovery made in 2006, when scientists succeeded in converting differentiated cells into stem cells.
Differentiated cells are cells that acquired their definitive cell type and function. These are the “somatic” cells that build up our organs and tissues. Examples of differentiated cells are liver cells, skin cells, brain cells, muscle cells, and so on.
Stem cells are undifferentiated cells. They are undifferentiated because stem cells don’t have a cell identity: they are not cartilage, gut nor fat cells. They are the cells that produce differentiated cells, which are the main cells that build up our body. Stem cells generate skin cells, bone cells, fat cells, and so on (learn more about stem cells here).
First, there were embryonic stem cells
Until 2006, it was very difficult for scientists to use “pluripotent stem cells”. This was a big problem, given pluripotent stem cells are very powerful stem cells that can create all the different types of cells found in an adult (we explain more about pluripotent stem cells here).
Until then, the only way to get pluripotent stem cells was by using embryonic stem cells.
Embryonic stem cells are derived from early-stage embryos that are 3 to 5 days old and consist of only about 150 cells.
A while ago, using embryonic stem cells was considered by some as controversial, given that embryos had to be “sacrificed” to get these stem cells.
However, embryonic stem cells are derived from very early-stage embryos which are only a few days old, and which would otherwise be discarded anyhow. These embryos are created during in-vitro fertilization procedures, in which excess embryos are produced while only one embryo can be implanted in the womb. Instead of discarding them, the extra embryos are donated for research (of course with informed consent of the donors).
Embryonic stem cells are then derived from the embryos. As mentioned before, these embryos consist of a handful of cells – they don’t have a brain, given they are just a small microscopic ball of mainly undifferentiated cells without any neurons.
For people who still find discarding early-stage embryos difficult to stomach, it could be interesting to know that in general about 60% of human embryos are spontaneously aborted, mostly before the mother or doctor knows one is pregnant.
And another 10 to 15 percent of pregnancies result in later stage miscarriages, which then often do not go unnoticed.
This means that around 70 to 75 percent of fertilized eggs do not result in a live birth in humans. Humans are not good at getting pregnant and carry children (R). Aborting embryos is what happens most of the time in our species.
The fact that these embryonic stem cells had to be derived from early-stage embryos at fertility clinics made it hard to come by them, and it was made even harder given various laws and procedures were installed making it difficult to use these cells. Luckily, a major scientific breakthrough solved this issue.
And then there were induced pluripotent stem cells
In 2006, two Japanese scientists (Shinya Yamanaka and Kazutoshi Takahashi) found a way to make stem cells without having to use embryos (R).
They could simply take almost any cell of one’s body (like a skin cell) and then convert this differentiated somatic cell into a pluripotent stem cell. Such reprogrammed cells are called “induced pluripotent stem cells” or iPSCs.
This is quite amazing, showing that many of the cells of our body still have the potential to be changed into a pluripotent stem cell, which then can create many different types of differentiated cells.
How did they do this? They exposed differentiated somatic cells (like skin cells) to a few specific proteins (called “transcription factors”), which then reprogrammed those cells back into stem cells (R,R).
These transcription factors famously are Oct4, Sox2, Klf4 and Myc. These 4 proteins are also called “Yamanaka factors” (or OSKM). Another combination of proteins for epigenetic reprogramming is OSNL (Oct4, Sox2, NANOG and LYN28) (R).
This was of course a huge discovery. Now scientists didn’t have to acquire stem cells from early-stage embryos, they could derive stem cells from almost any kind of somatic cell that is reprogrammed into a stem cell. Also, iPSCs are not surrounded with as much controversy as embryonic stem cells. Additionally, these stem cells can be made of cells from the patient, leading to no or much less immune rejection. After all, iPSCs can be made from one’s own cells, not from the cells from someone else.
No wonder that Professor Yamanaka almost instantaneously became a scientific celebrity, and was awarded the Nobel Prize around five years after his discovery, which is very fast in Nobel Prize terms (some scientists have to wait their entire life to get it) (R).
But what has this discovery to do with aging?
Yamanaka factors rejuvenate cells
It was found that when old cells are reprogrammed into stem cells, they become young again. Fascinatingly, all kinds of aging-related damage becomes undone during this reprogramming process: old, dysfunctional mitochondria start to work properly again, the protein mess that accumulated in old cells disappears, telomeres are lengthened again, DNA damage is repaired, and so on.
In fact, scientists succeeded in reprogramming normal, differentiated cells of people older than 80 years into young, healthy stem calls again using Yamanaka factors (R). This is remarkable, given it shows the enormous regenerative capacity of the human body to undo the large amounts of damage that accrued during aging. It seems that cells can remember, and know how to repair damage and become young and fresh again.
Given that these Yamanaka factors could rejuvenate cells, scientists wanted to test if they could also rejuvenate whole organisms this way.
So when they upregulated Yamanaka factors in mice, the mice… died. But this should not be very surprising. Too much Yamanaka factor activity causes cells to dedifferentiate too much: they become stem cells or too stem cell-like, losing their original identity and function. Liver cells, kidney cells, gut cells, brain cells and other cells become stem cells again, or very stem-cell like, causing organs and tissues to malfunction. It also caused teratomas in the mice, which are tumors consisting of a mixture of different types of tissue, like hair, bone, muscle and even teeth. It was as if cells lost their mind about what they were, or what they needed to become: a hair cell? A neuron? Something else?
So continuously upregulating Yamanaka factors in animals was not a very good idea.
But then scientists found out that only now and then activating Yamanaka factors was a better approach. Increasing the production of these proteins in mice in a cyclical way rejuvenated them.
More precisely, if cells produced Yamanaka factors for 5-days, followed each time by a break of 2 days, the mice seemed to become younger again: their organs could regenerate better, they had more and healthier stem cells, their cells could better withstand various stressors (like toxins), and in an aging-model mice lived longer (R).
So not continuous but cyclical activation of Yamanaka factors can have a rejuvenating effect. It could partially reverse aging in these mice, making them younger again (R,R).
How do Yamanaka factors rejuvenate cells?
Scientists do not yet know how Yamanaka factors exactly can rejuvenate cells. We do know they are transcription factors, which means these proteins latch onto DNA and activate dozens of other genes.
Also, Yamanaka factors have strong epigenetic effects. They reprogram the epigenome of cells into a more younger, stem-cell-like, regenerative state.
Interestingly, later studies showed that using fewer Yamanaka factors (only three factors instead of four) also led to rejuvenation while not having harmful effects in mice (so not forming teratomas or causing other problems) (R). So the fear that Yamanaka factors could cause cancer (by forming teratomas for example) got more subdued by this study.
Other Yamanaka factors have also been found, such as a combination called “7F”, consisting of 7 proteins that can also reprogram cells (Jdp2, Jhdm1b, Mkk6, Glis1, Nanog, Essrb and Sall4).
As mentioned earlier, Yamanaka factors induce “pluripotency”: they can make stem cells that are pluripotent, meaning they can generate hundreds of different types of cells. However, some proteins that induce “multipotency”, like Msx1, also seem to be able to rejuvenate cells (R) (“multipotent” stem cells can only give rise to a few different types of cells – I explain the difference between multipotent and pluripotent stem cells here).
While Yamanaka factors are being used in thousands of labs all over the world, there are still various hurdles to overcome. The reprogramming process is not very efficient: most cells are not reprogrammed or rejuvenated when exposed to Yamanaka factors.
However, this reprogramming process can be improved in various ways, for example by reducing a protein called Mbd3 (R), or by activating specific regions in the DNA, like the EEA motif (embryo genome activation-enriched Alu-motif) or by expressing miR-302 (R). Scientists are discovering many more methods to improve the reprogramming.
Scientists are also looking into ways to decouple the rejuvenation process from loss of somatic identity (the latter happens when normal somatic cells dedifferentiate too much and become too stem-cell like) (R).
Some scientific leaders in the field of epigenetic rejuvenation are Alejandro Ocampo, Manuel Serrano, Juan Carlos Izpisúa Belmonte and David Sinclair.
In conclusion, epigenetic reprogramming via Yamanaka factors showed that old cells, and even entire organisms, can be rejuvenated, and that aging is a reversible process.
Various companies, like Turn Therapeutics, Altos Labs (R,R), Life Biosciences and others are currently developing treatments based on this approach to rejuvenate or repair tissues in humans.
2. Reversing aging via clearing senescent cells
Another way to partially reverse aging is by getting rid of the senescent cells that accumulate everywhere in our body as the decades of our lives pass.
As we discussed elsewhere, senescent cells are damaged cells that stop dividing but refuse to die, secreting various harmful substances that damage healthy surrounding cells.
Senescent cells in the skin contribute to wrinkles, senescent cells in our blood vessels secrete proinflammatory substances that spread to every nook and corner in our body, and senescent cells in the liver, lungs or brain impede the proper functioning of these organs.
In one study, scientists cleared senescent cells by giving mice a peptide that triggered senescent cells to commit suicide (R). This approach restored fur density, fitness and renal function in fast-aging mice and in naturally-aging mice. The mice not only looked younger, but also behaved younger (e.g. being more explorative or cognitively astute) and their organs functioned better.
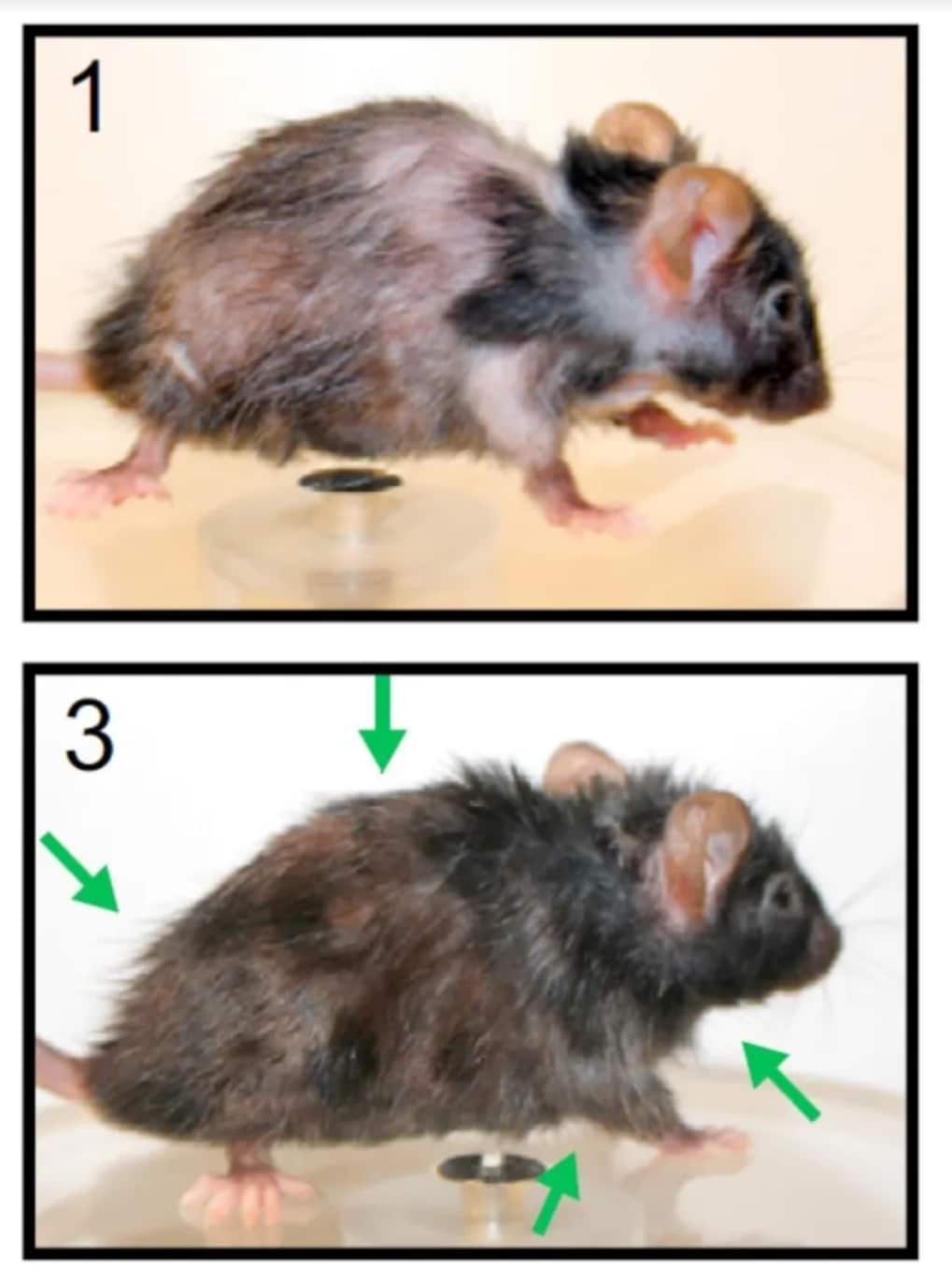
The mouse at the top is old, having gray fur, bald spots and an osteoporotic back. After clearing senescent cells, the same mouse looks younger (image below), with increased black fur, less bald spots and a reduced osteoporotic back (Picture by Peter De Keyser, Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging, Cell, 2017)
Scientists are trying to find various ways of clearing senescent cells. We explain more about this in the chapter in the part about “Causes of Aging: Senescent Cells”.
By clearing senescent cells, scientists showed that it’s possible to make old organisms younger again. Their organs and tissues can regenerate, function and repair themselves better again.
Very likely, we will go to a future where people will regularly receive treatments to clear away senescent cells. Destroying senescent cells in cartilage could improve osteoarthritis, destroying senescent cells in the skin could reduce wrinkles, and getting rid of senescent cells in the lungs or liver could improve the function of these organs again.
3. Reversing aging via growth hormone, DHEA and metformin?
In a study run by biologist Greg Fahy which got a lot of attention people’s immune system and body seemed to be rejuvenated by taking a combination of three substances.
Participants were given a cocktail of two hormones (growth hormone and DHEA) and a drug (metformin). After one year, their epigenetic age was reduced by 2.5 years compared to the start of the study (R,R).
However, some reservations about this study can be made.
1. Growth hormone and male hormones accelerate aging and shorten lifespan
First, in this drug cocktail, two substances were used that have been shown in many other studies to actually reduce lifespan and accelerate aging. These substances are growth hormone and DHEA, a male sex hormone.
Too much growth hormone, or activation of growth hormone-related pathways, has been shown to accelerate aging in thousands of scientific studies (R,R,R). In a nutshell, growth hormone induces growth, and too much growth in many cases accelerates aging.
Male sex hormones, especially testosterone and dihydrotestosterone, have also been associated with shorter lifespans and accelerated aging (R), whereas castration (which leads to a drastic reduction in sex hormones) extends lifespan in multiple species, including monkeys and humans (R,R). DHEA is a precursor to male sex hormones. In one study in which mice were given DHEA, these mice lived 25% shorter compared to the control group that didn’t receive DHEA (R) (Learn more here how male sex hormones, like testosterone, can accelerate aging).
Nonetheless, many people still tout the great “anti-aging” benefits of DHEA, claiming DHEA improves insulin resistance, bone density, libido, or reduces visceral fat. Often, people also feel better when taking DHEA.
However, these are all short-term effects (similar to testosterone by the way). In the long term, DHEA might accelerate aging given it’s a potent precursor of male sex hormones.
What also contributes to confusion around DHEA, is that various studies show that higher levels of DHEA correlated with improved health in humans. However, this could be an association, not a causative effect: often, people who are healthier have higher DHEA levels, given sickness and accelerated aging can cause a decline in the production of DHEA.
That male sex hormones accelerate aging should not be surprising in the light of biology and evolution. Firstly, the “disposable soma theory” states that reproduction – and associated sex hormones – are at the expense of longevity given both processes require significant amounts of energy, making then when investing energy in reproduction less energy can be spent on maintenance of the body to slow aging. There is also the famous “antagonistic pleiotropy theory” which states that what can make you strong and fit when you are young could make you age faster later on. So people who produce lots of male sex hormones when young can be strong and muscular, and have stronger bones and more libido so they can reproduce more, but at this expense they age faster; however, nature does not mind this given in prehistoric times most people didn’t had the chance to become old; many died quite early to external causes of death, like violence, disease or starvation.
Especially growth hormone is well known to be a pro-aging hormone, and is known to have serious side effects, like an increased risk of cancer (cancer cells are cells that exhibit “uncontrolled growth”), swelling in arms and legs (edema), impingement of nerves (like carpal tunnel syndrome), increased insulin resistance, and type 2 diabetes. To mitigate the risk of type 2 diabetes, metformin, an anti-diabetic drug was added to the cocktail.
2. Metformin could have been responsible for the beneficial effects
The fact the researchers added metformin is important, given that metformin is a drug with potential longevity and anti-aging effects. It could be that the metformin used as part of this drug cocktail was an important driver of the rejuvenation effects, and not DHEA and growth hormone. And that perhaps when the growth hormone and DHEA were left out, the rejuvenation effect would be more pronounced.
3. Only a short term study
Furthermore, it could also be that this apparent “rejuvenation” is only short-term, and that in the long term (e.g. when given for many years or decades) regular exposure to growth hormone and DHEA accelerates aging, despite the metformin in the cocktail.
4. No control group
Arguably the biggest shortcoming of this study was that it didn’t have a control group.
This means it could be that epigenetic age was reduced due to the placebo effect.
The placebo effect can be very strong, and just the thought that you are taking drugs that could have rejuvenating effects could improve health and epigenetic age.
5. Epigenetic clocks are not yet that accurate
To determine epigenetic age, in this study four different epigenetic clocks were used, namely the Horvath 2013 DNAm Age clock, the Hannum DNAm Age clock, the Levine DNAm PhenoAge clock, and the Grimage clock. Some of these clocks are not that accurate unfortunately to determine lifespan interventions in humans.
Additionally, the epigenetic age was measured by looking at peripheral blood mononuclear cells (PBMN cells), which are white blood cells (mainly T cells). However, this doesn’t necessarily say a lot about rejuvenation of other cells and tissues, like for example brain cells, kidney cells or heart cells.
However, we do know that there is a correlation between the epigenetic age of peripheral blood mononuclear cells and general health and longevity. Nonetheless, this correlation still can be skewed. For example, if the immune system is more “renewed” due to an influx of new, fresh white blood cells due to a recent infection for example, or is more “older” because lots of old T cells that fought previous infections a long time ago are still circulating, this will make the epigenetic age incorrectly “younger” or “older”. Scientists are working on mitigating this effect.
In this regard, another explanation for the “rejuvenation” effects of this cocktail could be that growth hormone and DHEA increase the “growth” and metabolism of the thymus, so it churns out more new T cells. These are young, fresh T cells, and given the epigenetic clocks measure the epigenome in T cells, the body “looks” rejuvenated.
However, in the long term, this throttled up growth by growth hormone and DHEA can actually make the thymus wear out faster, and make the body in general age faster (the growth hormone and DHEA act on all our cells).
Such an effect would only become noticeable after many decades, and given almost no human study takes so long, it remains difficult to see whether this therapy could actually increase lifespan.
6. People also took zinc and vitamin D
One final thought: in this trial, participants also received a high dose of zinc (50 mg) and vitamin D (3,000 units per day).
We know that zinc and vitamin D have significant positive effects on the immune system and on the aging epigenome; so perhaps some (or even a large part) of the rejuvenation could have come only from this intervention. Ideally, there would also have been a control group that also did receive zinc and vitamin D, but not the growth hormone, DHEA and metformin cocktail, to disentangle these effects.
To make a long story short, at this stage, I would be careful using well-known, pro-aging hormones like growth hormone (especially), and male hormones like DHEA (likely) for rejuvenation – unless one is significantly deficient in these hormones, but then it would be better to find out the primary cause of this deficiency.
4. Reversing aging via lifestyle
To substantially reverse aging, we will need cutting edge pharmaceutical and biotechnological approaches, like epigenetic reprogramming, targeted senescent cell clearance, mitochondrial rejuvenation, and so on.
However, currently, lifestyle is the best and most powerful approach we have to slow down and even partially reverse aging. This is at least what studies are showing; researchers and doctors find that putting people on specific diets and supplements, and making them exercise and sleep properly can rejuvenate people, at least according to epigenetic clocks.
Epigenetic clocks look at patterns in the epigenome (more specifically, they look at hundreds of base pairs in the DNA to see if the base pair is methylated or not).
Depending on the pattern, one can be younger or older biologically than one’s chronological age. Likely, the best way to measure aging (your real biological age, as opposed to your chronological age) is via epigenetic clocks, which are quickly becoming precise and accurate (learn more about epigenetic clocks here).
Lifestyle interventions can partially reverse epigenetic clocks
In one study, patients followed a healthy diet, took specific supplements and exercised for 8 weeks. After this intervention, their epigenetic age was reversed by almost 2 years (R).
Various other studies show that when people adhere to a healthy diet, or do regular exercise, reduce stress, moderate alcohol consumption, their epigenetic ages can be reduced, making them younger biologically (R,R).
There are various specific foods that can slow down aging, and perhaps even partially reverse it. Many of these foods act on the epigenome too. Make sure you consume lots of the following foods:
- Lots of vegetables, especially broccoli, cauliflower, spinach, kale, sprouts
- Legumes, such as beans, peas and lentils
- Less bread, potatoes, pasta and rice: replace as much or entirely with vegetables, legumes, mushrooms (e.g. so cauliflower mash instead of potato mash or mushrooms instead of pasta)
- Red and blue fruits like blueberries, blackberry, strawberries and raspberries
- Little or no red meat (beef, pork, mutton, veal). Replace more with white meat (poultry) or fatty fish
- Little or no alcohol
- Lots of herbs (parsley, oregano, basil, curcumin, ginger, etc.)
There are also various anti-aging supplements that could improve your aging process and often can help to maintain the epigenome, such as:
- Low-dose lithium
- Alpha ketoglutarate
- B vitamins
- NMN
- And many others
I list the best longevity supplements here.
Of course, various healthy lifestyle methods can also be beneficial for your epigenetic, biological age, such as:
- Proper sleep (enough sleep and going to bed at the same hour)
- Not smoking
- Regular exercise
- Stress reduction
Learn more here on how to slow aging.
In conclusion, our lifestyle is currently the best and most powerful method we have to not only slow down aging, but even partially reverse it.