Rapamycin (also called sirolimus or rapamune) is one of the most well-known, researched, and promising drugs for anti-aging and longevity.
According to various experts, including Professor Matt Kaeberlein, rapamycin is currently the best drug we have for longevity, significantly better than other drugs, including metformin (more about this drug later).
Rapamycin was discovered on Easter Island around the 1970’s; the drug is named after “Rapa Nui”; the name for this island by its original inhabitants. Rapamycin is a complex molecule derived from bacteria that live in the soil, called Streptomyces hygroscopicus (R).
In fact, the typical smell of fertile land after rain comes in part from volatile compounds produced by Streptomyces bacteria.
Around twenty years later, rapamycin was approved by the American Food and Drug Administration (FDA) to prevent organ rejection in kidney transplant patients. Since then, rapamycin has been approved for various other indications, such as liver transplantations, perivascular epithelioid cell tumors (PEComa, a rare tumor that forms in the soft tissues of the intestine, stomach, lungs and other organs), and lymphangioleiomyomatosis, a rare lung disease in which cysts grow in the lungs, lymph nodes and kidneys.
Stents (little coils that go into narrowed, atherosclerotic arteries to open them up) are also coated with rapamycin to prevent them from growing over by tissue when they are put into an artery to widen it.
Rapamycin has been researched for decades, and numerous studies show that this drug can extend lifespan in various species (R,R,R,R), not only in unhealthy or diseased animals, but more importantly, also in normal, healthy animals (R,R,R).
For example, rapamycin increased lifespan in male mice by 23% and 26% in females when started in relatively young mice at 9 months of age (the average lifespan of lab mice is 2 years, so 9 month old mice would be around 35 years in humans) (R).
When started later in life (in 600-day-old mice), rapamycin still had an effect: it increased maximum lifespan by 9% in males and 14% in females (R).
However, some scientists believe that the doses of rapamycin used in most studies are too low, and that even stronger longevity effects could be realized if the doses would be higher (R).
For example, in one study three different doses of rapamycin were administered in young to middle-aged mice (9 month-old) mice (the doses were 4.7 parts per million (ppm), 14 ppm and 42 ppm; ppm means one millionth of a substance, like 1 mg of rapamycin per kg of food, or 1 mg of rapamycin per liter of drinking water).
The lowest dose (4.7 ppm) increased medium male lifespan by 3% and medium female lifespan by 16%. A higher dose (14 ppm) increased medium lifespan in males by 13% and 21% in females. The highest dose (42 ppm) increased medium lifespan in males by 23% and 26% in females (R,R):
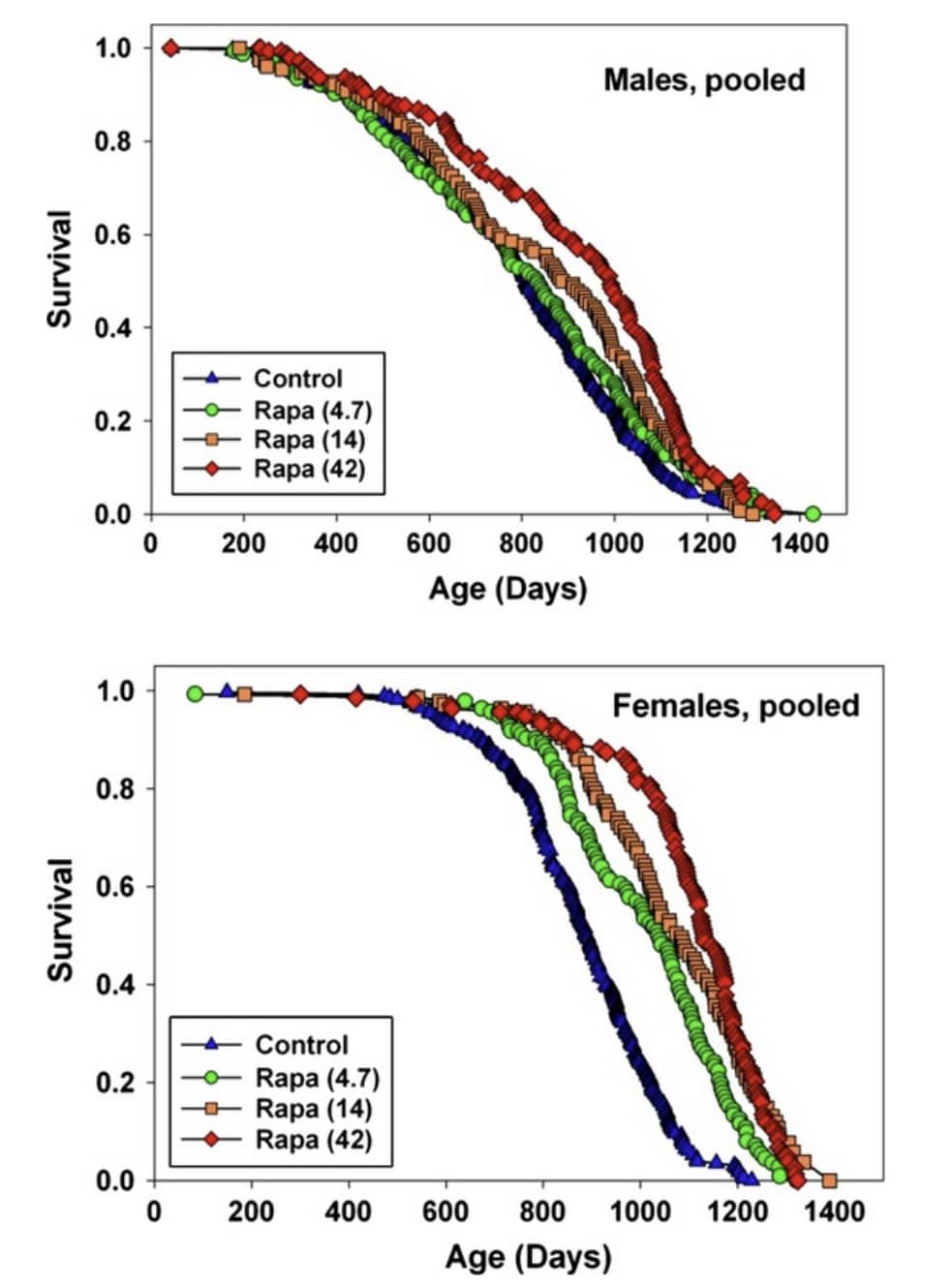
Higher doses of rapamycin lead to more life extension
That’s why some researchers recommend using higher doses, like 42 ppm, 140 ppm and even 420 ppm for new longevity mice studies (R).
Other studies, in which higher doses of rapamycin were used also showed larger effects. For example, in one study old mice (20-22 months of age) were injected every other day during 6 weeks with rapamycin (4 mg/kg). The drug was injected intraperitoneally, meaning injected in the abdominal cavity.
Intraperitoneal injection leads to a fast, very high peak of rapamycin in the blood, much higher than when rapamycin is given orally. At the end of the study, 80% of the mice that got rapamycin were alive, compared with only 20% of the control group (R). So mortality was 4 times lower in the rapamycin group:
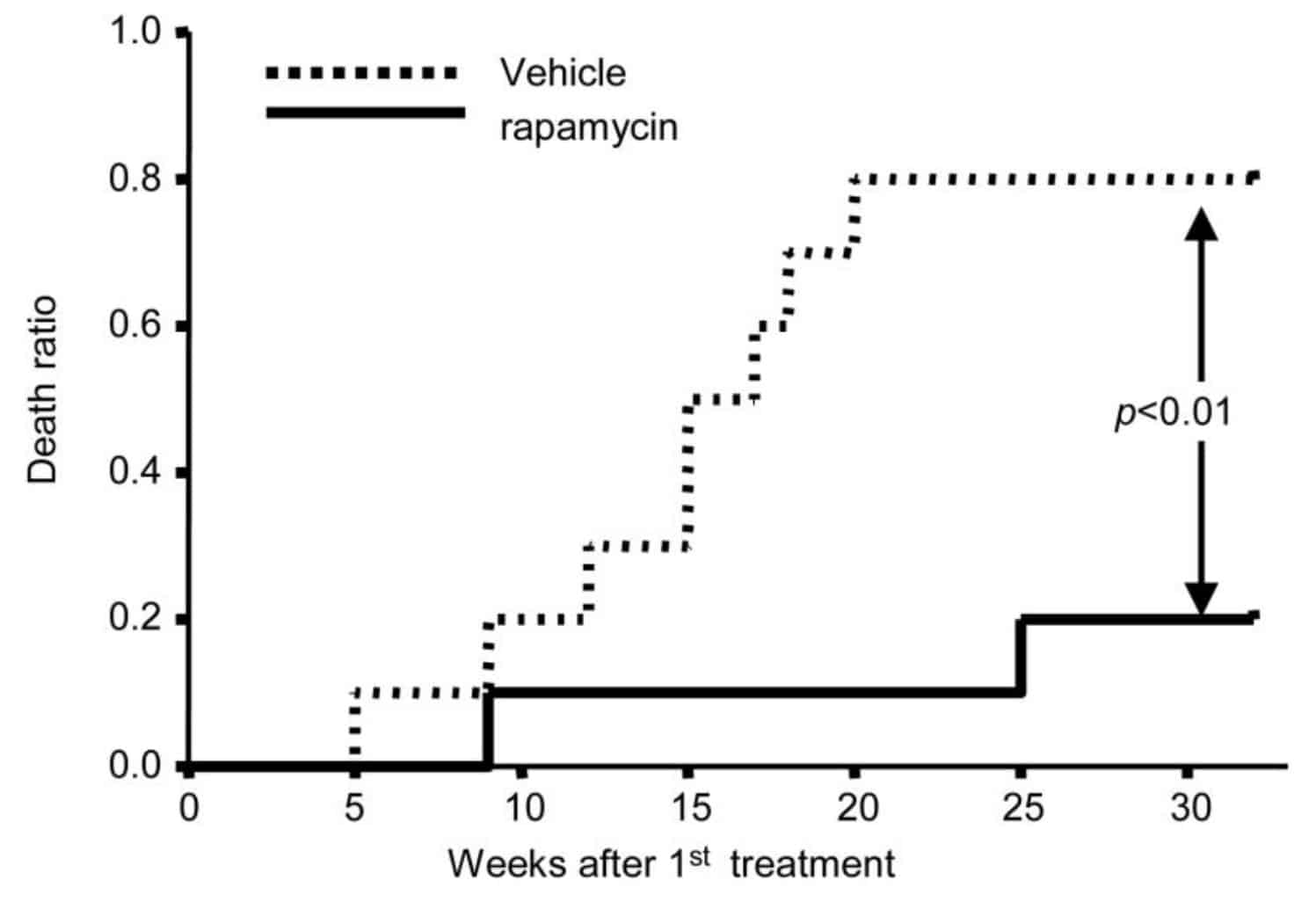
Considerably less mice that received intraperitoneal injections of rapamycin every other day (4 mg/kg) died 30 weeks after treatment (black line) versus mice that didn’t receive rapamycin injections (dotted line)
In another study mice were injected with rapamycin (1.5 mg/kg) starting at 2 months of age intermittently: they received an injection 3 times per week for two weeks out of every month. At 800 days of age, 54% of the treated animals were alive, compared to 36% of the control animals. At day 900, the results were even more impressive: 31% of the mice that received rapamycin were alive, while only 10% of the control mice were alive (R).
One study examined the impact of rapamycin in obese mice. Mice were put on an unhealthy high-fat diet. After 2 years, 100% of the mice that received weekly injections with rapamycin were still alive, while more then 60% of the untreated mice had died (R):
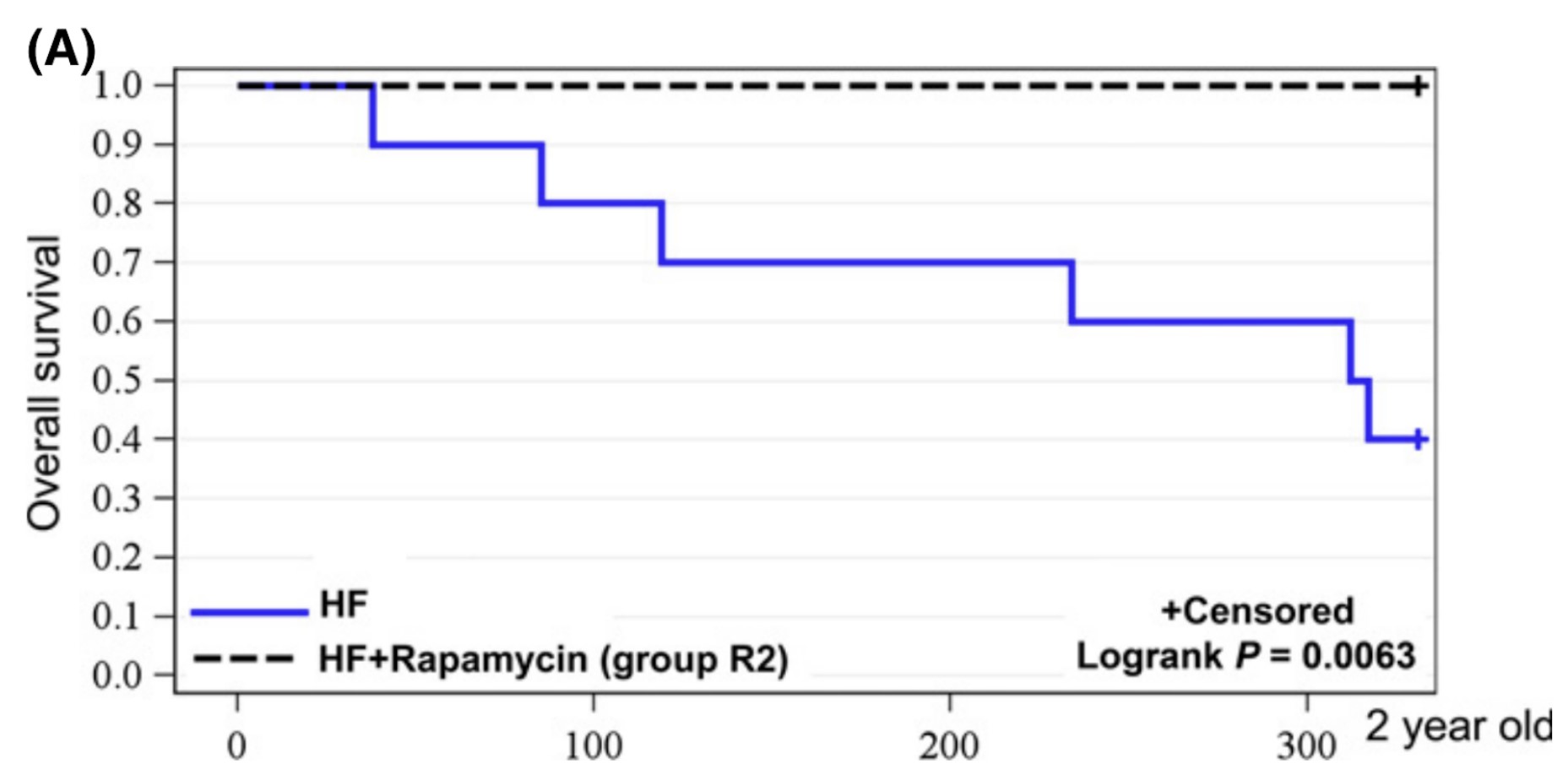
Mice are put on an unhealthy high-fat diet. After two years, mice that received weekly rapamycin injections are all alive (black dotted line) while about 60% of mice without rapamycin died (blue line)
This does not necessarily mean that humans have to take very high doses of rapamycin. But this does show that results in mice could perhaps be even more impressive when the doses would be higher.
This is why various rapamycin longevity researchers who take rapamycin themselves believe that the doses should at least be sufficiently high enough (I will discuss rapamycin doses for humans later on).
But the dose should not be too high of course. Too high a dose of rapamycin, especially when taken continuously, can have various significant side-effects (which I will also discuss later on).
On the other hand, some fears of rapamycin are exaggerated, like that rapamycin impairs the immune system, increasing the risk of infections (I explain more about this here), or could lead to increased glucose levels and type 2 diabetes (I explain more about rapamycin and diabetes risk in this article).
Rapamycin and cancer
Besides extending lifespan, we see that rapamycin can improve various aging-related diseases, including cancer. This makes sense, given rapamycin curbs various growth pathways in cells, and cancer cells are exhibiting uncontrolled growth.
For example, rapamycin can extend the lifespan of cancer-prone mice (R,R,R):
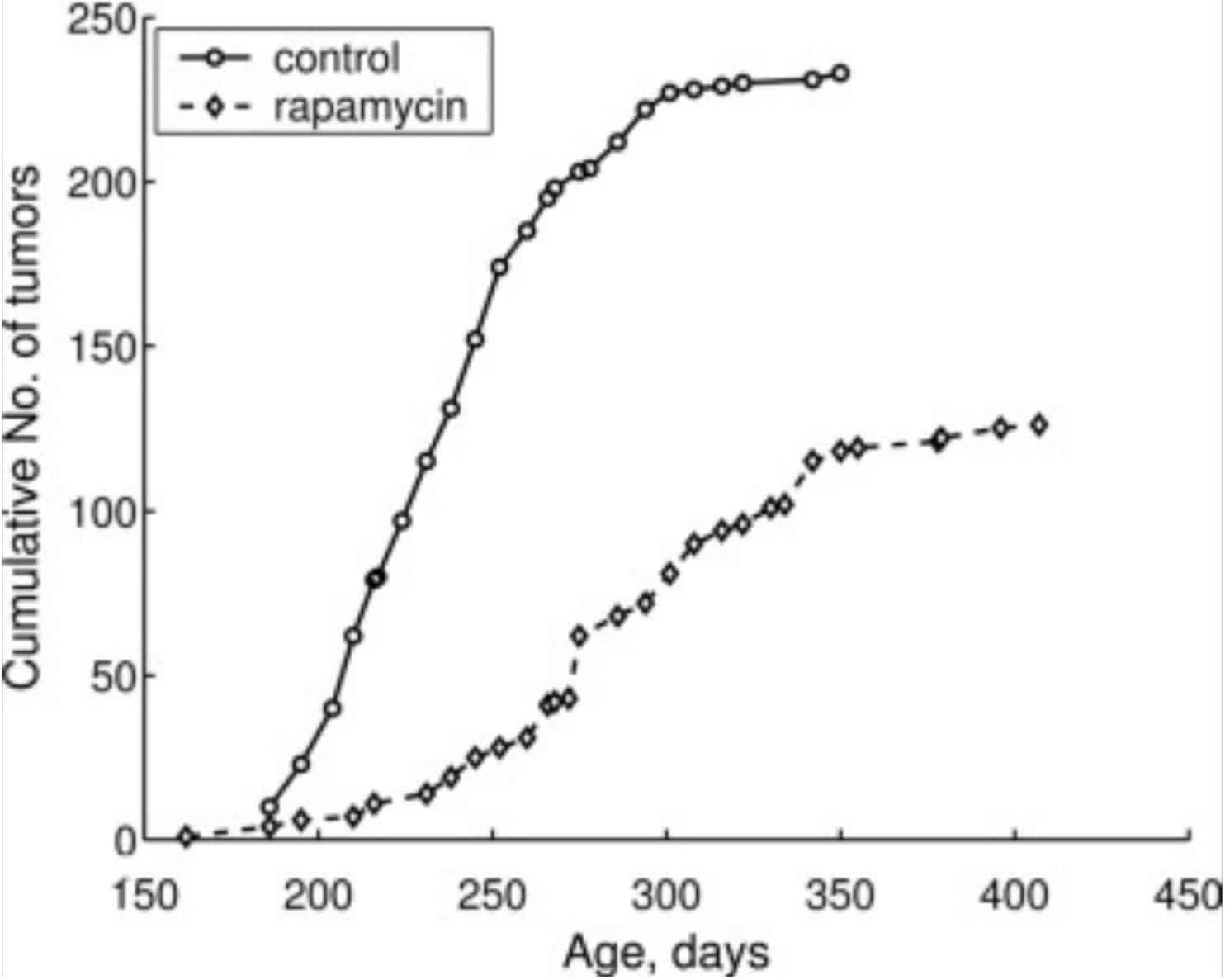
Rapamycin could also prevent or slow down tumor growth in various other rodent studies (R,R,R).
Rapamycin and heart disease
Rapamycin and similar drugs could also improve heart health.
Mice fed a high-cholesterol or high-fat diet had 48% less plaques in one study (R), and 44% to 48% less plaques in another study (R). “Plaques” are sticky, hard parts forming in our blood vessels, contributing to atherosclerosis.
Cardiac hypertrophy could also be significantly reduced in mice (R,R,R). Often, when the heart has to pump against increased resistance (as can happen when our arteries become more stiff during aging) the heart muscle starts to swell, becoming “hypertrophic”, which impedes the proper contraction and functioning of the heart.
Rapamycin could also improve heart function during aging in mice (R,R,R).
How does rapamycin work? How can it slow aging and extend lifespan?
Rapamycin can slow aging and extend lifespan in various ways.
1. Rapamycin is an mTOR inhibitor
First, rapamycin is an mTOR inhibitor. mTOR is a protein found in our cells. It’s an important “nutrient” sensor which senses nutrients, mainly amino acids. If there are a lot of nutrients like amino acids present in our blood and cells (because we ate a big steak for example, which consists of proteins which are made up of amino acids), this activates mTOR. mTOR then revs up the production of protein (mainly, but also of DNA and lipids), metabolism and growth in our cells.
This makes sense. If there are a lot of nutrients (e.g. amino acids) available, this means there are a lot of building blocks and energy for the cells, so the cells rev up their metabolism, growth and work harder.
However, this activation of metabolism and growth accelerates the aging of cells. For example, cells start to produce more proteins, which can clump together faster, contributing to aging (accumulation of proteins is one of the hallmarks of aging).
Another consequence of having lots of nutrients available, is that cells will maintain themselves less well. They let themselves go. Why should they be frugal, and painstakingly recycle their components and maintain and repair themselves, when there are enough building blocks and energy available? No need to skimp on repair, recycling and maintenance when there are plenty of resources. Compare it with money; some people who have suddenly lots of money splurge on all kinds of things they don’t really need, spending it quickly, living the fast life. On the other hand, people who have little money will live more frugally, be careful what to spend, and will try to recycle stuff for their home or rather repair it than buy new things.
So how does mTOR activation cause less maintenance? It suppresses autophagy for example, which is an important maintenance and recycling process. Autophagy is the breakdown (digestion) of proteins and other cellular waste material. mTOR-induced suppression of autophagy, which is not a good thing, causes more buildup of (damaged) proteins, old and damaged cell components and other problems. If you eat lots of protein, especially animal-based protein, mTOR is activated and autophagy is suppressed.
The opposite happens when we are eating little (e.g. during fasting): autophagy is then upregulated, which cleans up the cell and increases maintenance and lifespan. The cell has to use its resources wisely because it doesn’t know when there will be food again.
Rapamycin can also induce autophagy independent of mTOR. Rapacymin stimulates an important channel on the lysosomes (TRPML1), which activates the lysosomes (R). The lysosomes are small vesicles in our cells which break down proteins and old or damaged cell components – you can consider them as the incinerators of the cells (I explain more about lysosomes and autophagy in the earlier chapter the hallmarks of aging, namely a decline in proteostasis).
2. Rapamycin and mitochondrial health
Rapamycin could also be promising to treat mitochondrial diseases.
Rapamycin has been shown to improve mitochondrial health, both in normal mice (R) and in mice with serious mitochondrial diseases (R,R,R,R).
In one mitochondrial disease model, called Leigh’s syndrome, rapamycin was given to see if it could improve survival of mice with this disease. Leigh’s syndrome is a very serious mitochondrial disorder caused by a defective protein in the “respiratory chain”. This is the “chain of proteins” through which electrons flow (coming from the food you eat, which are then donated to the oxygen molecules you breathe in to form water).
The mitochondrial respiratory chain generates nearly all the energy of the cell, and thus for life to sustain itself. In Leigh’s syndrome, a protein in this chain is not working properly, severely impeding the ability of cells to produce energy. In these mice, rapamycin extended lifespan up to threefold (R).
How could rapamycin improve mitochondrial health?
For one thing, rapamycin can upregulate lysosomal function. Lysosomes are small cell components which break down cellular waste and even whole cell components, like the mitochondria. Each cell can contain hundreds or more mitochondria, which provide the energy cells need to function. These mitochondria, when they are too damaged or worn out, need to be broken down and replaced by new mitochondria. Mitochondria are broken down in the lysosomes, the little incinerators of the cell.
Rapamycin induces the lysosomes to break down mitochondria so that worn-out mitochondria are more replaced with fresh and better functioning mitochondria. Rapamycin can also activate lysosomal biogenesis (the creation of more lysosomes), which is a good thing given cells then get cleared out and maintained better by additional lysosomes (R,R).
3. Rapamycin has epigenetic effects
Rapamycin also impacts the epigenome, and can reduce epigenetic age (R,R). The epigenome determines which genes are switched off and on in our cells, and during aging this mechanism goes awry. Rapamycin can improve the epigenome in a beneficial way, for example by inducing the expression of beneficial genes (R,R,R).
4. Rapamycin improves the microbiome
The gut microbiome are all the bacteria in our intestines. These 40,000 billion bacteria have a large impact on our health and well-being by processing and secreting thousands of substances, of which many enter our bloodstream and impact our immune system, metabolism, and brain.
However, during aging, the microbiome deteriorates, leading to bacterial overgrowth, increase of “virulent” (bad) bacteria, and damage to the gut lining (“leaky gut”) for example.
Rapamycin can positively impact the bacterial diversity in the gut, or inhibit bacterial activity. In one study in mice, rapamycin increased the mount of segmented filamentous bacteria, which are “good” bacteria that do not tend to secrete unhealthy substances and that strongly cling onto the gut wall, which might strengthen the gut lining and positively impact the gut immune system (R).
Interestingly, another well-known longevity-candidate, metformin, has also been shown to significantly impact the microbiome, by also modulating or tempering bacterial activity in the gut.
Other effects of rapamycin on health
Given these and other effects of rapamycin on the body, it’s not surprising that rapamycin can improve stem cell health (R) and reduce senescence (R), and extend lifespan.
Learn more about rapamycin:
- Does Rapamycin Cause High Glucose, Lipids and Diabetes?
- Does Rapamycin Increase Infection Risk?
- How Does Rapamycin Work? Overview of Rapamycin Lifespan Extension Studies
- Rapamycin for Anti-Aging and Longevity
- How does rapamycin work? How can it slow aging and extend lifespan?