What causes us to age, and to get all these aging-related diseases and symptoms, and to eventually die?
There are some fundamental, primary causes of aging, which then lead to secondary causes of aging.
Fundamental, primary causes of aging are:
- Epigenetic alterations
- Loss of proteostasis
- Mitochondrial dysfunction
- Genomic instability
- Telomere attrition & shortening
- Advanced Glycation End Products (AGEs) and protein modifications
These primary causes of aging lead to secondary aging mechanisms in our body, such as:
- Senescent cells
- Stem cell exhaustion
- Altered intercellular communication
- Deregulated nutrient sensing
- Other reasons why we age
Of course, various other aging mechanisms will likely be discovered in the future. But these mechanisms described here are a good start
1. Epigenetic Alterations
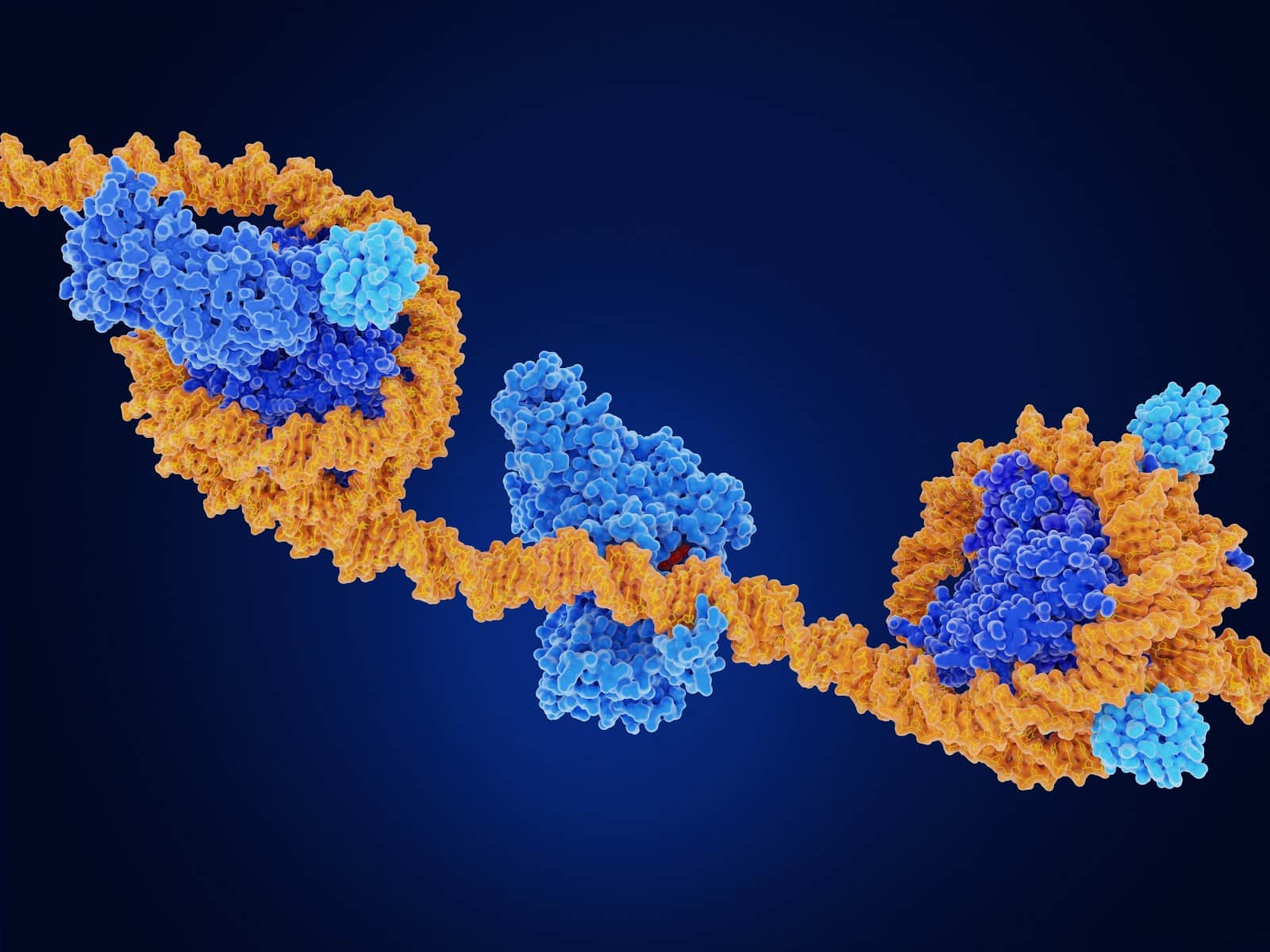
DNA strands (yellow) are wrapped around histone proteins (blue).
The epigenome is the machinery that surrounds our DNA and that determines which genes are active or silenced.
As we get older, the epigenome becomes more dysregulated, meaning various genes and other parts of our DNA become activated, while these should be silent, such as cancer and pro-inflammatory genes.
Vice versa, during aging, beneficial genes that should be active are switched off, like housekeeping and repair genes. This dysregulation of the epigenome causes us to age.
Learn more about how the epigenome is involved in aging here.
2. Loss of Proteostasis
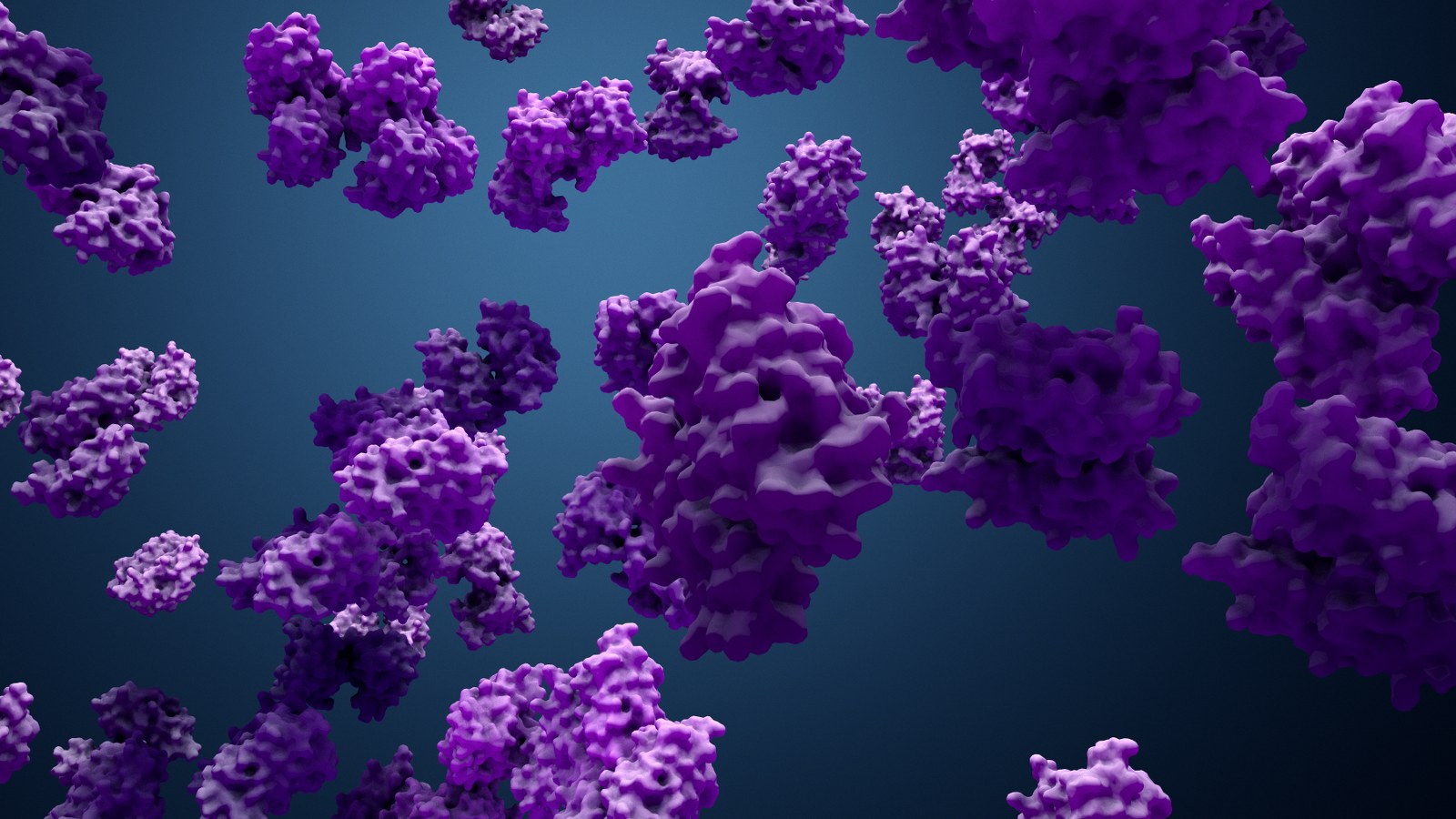
As we get older, proteins start to accumulate inside and outside our cells, hampering the functioning of our cells, causing us to age.
Proteins tend to stick together and form agglomerates, piling up in our body.
Normally, these protein aggregations are cleaned up via autophagy and other mechanisms, but during aging these mechanisms start to decline.
Protein accumulation plays a role in many aging-related diseases, such as Alzheimer’s disease, heart failure and hardening of the arteries.
Learn more about how proteins are involved in aging here.
3. Mitochondrial Dysfunction
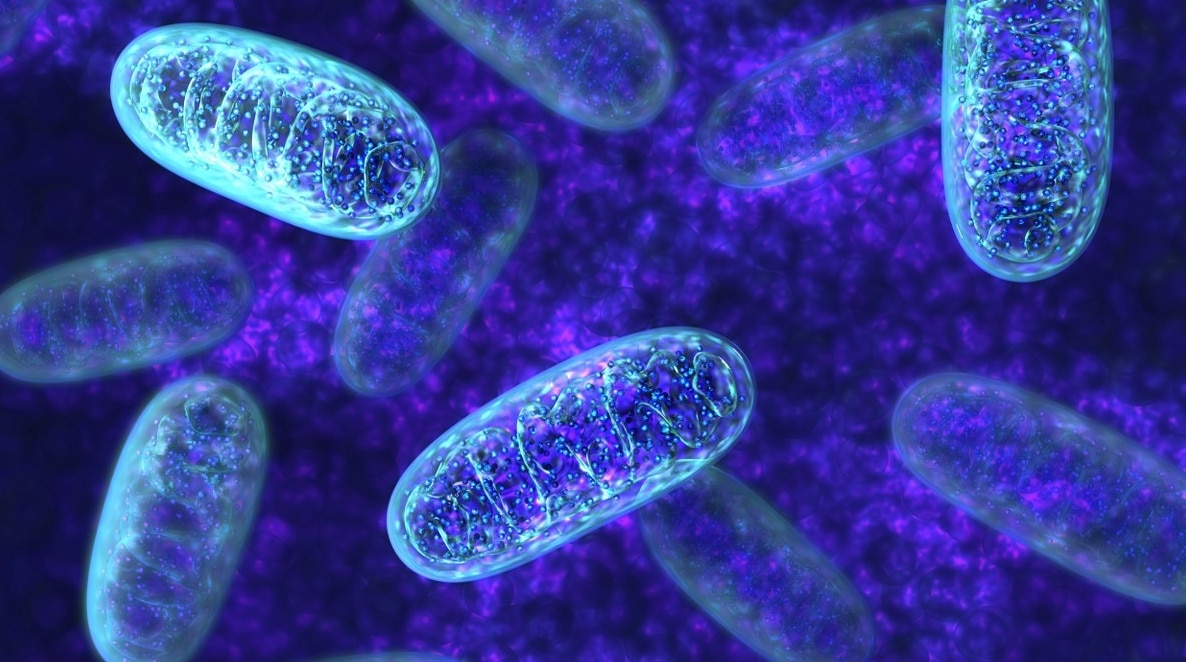
As we get older, the power plants of our cells, our mitochondria, decline.
Our mitochondria are very vulnerable to damage, given they are chemically very active (creating lots of free radicals and other damaging molecules) and need to multiply, leading to replication errors in the mitochondrial DNA.
This leads to less energy for our cells, and to the activation of various stress pathways and negative effects in our cells, causing them to age.
Learn more about how mitochondria contribute to aging here.
4. Genomic Instability (DNA Damage)
DNA gets damaged during aging.
Our DNA contains the instructions to build our body.
More precisely, DNA contains the instructions to build proteins, which build up our cells and carry out most of the functions in our cells.
During aging, our DNA becomes damaged by chemicals, free radicals, toxins, UV radiation, metabolites and by many other substances.
During cell division, damage also happens given the process of cell division is not perfect.
Additionally, rogue parts of our DNA (called transposons) jump around in our DNA, inserting themselves randomly in our DNA, disrupting and damaging our DNA.
Learn more about how DNA damage causes us to age here.
5. Telomere Attrition and Shortening
Telomeres are the ends of our DNA strands. They are often likened to the plastic caps on our shoelaces, which prevent shoelaces from unravelling. Somewhat similar, telomeres protect the ends of our DNA.
However, during each cell division, telomeres get shorter. Until they are so short they cannot properly stabilize and protect our DNA.
During aging, our telomeres not only become shorter, but also become damaged, which further stresses our cells.
There exist lots of misunderstandings about telomeres. For example, that longer telomeres lead to cancer. However, things are more complicated (and interesting).
Learn more about the roles of telomeres in aging here.
6. Advanced Glycation End Products (AGEs) and Crosslinks

As we get older, sugars bind to proteins, glycating and crosslinking them.
Sugar-based crosslinks (Advanced Glycation End products) link proteins together, making the tissues that are made of these proteins more stiff.
For example, crosslinking of collagen and elastin proteins in our skin and our blood vessels makes them more stiff, contributing to wrinkles and high blood pressure.
As we get older, other molecules also attach themselves to proteins, damaging our proteins, or making them targets for immune cells.
Learn more about sugar crosslinks, glycation and other damage to proteins here.
7. Senescent Cells
As we grow older, senescent cells arise everywhere in our body. These “zombie” cells are damaged cells that refuse to die. They cannot die and divide anymore, but they secrete many substances that damage normal, healthy cells.
Senescent cells in the skin contribute to wrinkles. Senescent cells in the blood vessels contribute to atherosclerosis, and senescent cells in our organs undermine their function.
Learn more about the role of senescent cells in aging here.
8. Stem Cell Exhaustion

Stem cells produce the cells that make up our body.
Specific stem cells continuously produce red and white blood cells. Stem cells in the gut build the cells that make up our gut, and stem cells in our skin and hair replenish our skin and make our hair grow (and give hair its color).
Besides continuously producing the cells that make up our body, stem cells also repair and maintain our tissues and organs.
However, during aging, stem cell function declines. Many stem cells also die off. This leads to our tissues being less replenished and maintained.
Learn more about the role of stem cells in aging here.
9. Altered Intercellular Communication
As we age, the communication between our cells starts to go awry.
Cells start to secrete proinflammatory and other unhealthy, damaging substances.
The milieu in which our cells bathe becomes “aged”, making our cells aged.
Learn more about how altered intercellular communication causes us to age here.
10. Deregulated Nutrient Sensing

When we get older, the intricate systems that regulate how we process nutrients start to go awry.
“Nutrients” are the foods we eat, or more specifically, the sugars, fats and amino acids (from protein) we consume.
When we are young, the body is still very good at processing nutrients. This explains why children and young adults often can eat lots or unhealthy food and still stay lean.
However, as we get older, our body is less able to process the sugars, fats and amino acids we consume.
This explains why as we age, we put on weight easier, and we tend to develop abdominal fat (“a beer belly”) and start to suffer from high cholesterol, high triglycerides, and (pre)diabetes.
Learn more about the role of deregulated nutrient sensing in aging here.
11. Other Reasons Why We Age
Of course, there can be other reasons why we age, and many other aging mechanisms will be discovered in the future.
We highlight some interesting ones here.